Iodoform Test for Alcohols
Or collect it over water and test it with a lighted spill. The information in this chart has been supplied to Cole-Parmer by other reputable sources and is to be used ONLY as a guide in selecting equipment for appropriate chemical compatibility.

Alcohols Advanced 9 Iodoform Test For Ch3ch Oh R Youtube
Add dilute sodium hydroxide solution drop wise until the brown colour of iodine is discharged.
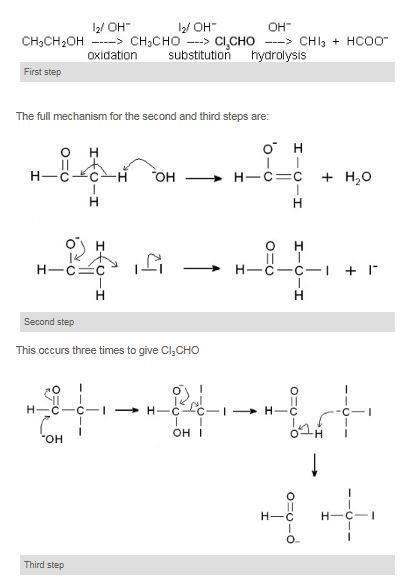
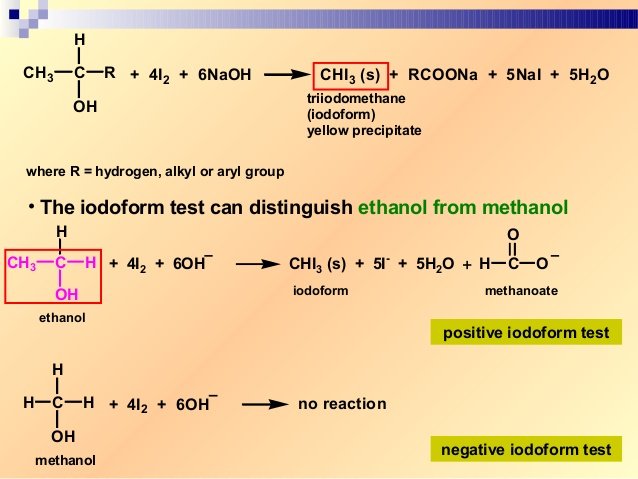
. Alcohols Phenols and Ethers Download CBSE Revision Notes for CBSE Class 12 Chemistry Alcohols Phenols and Ethers Alcohols. A spectacular practical demonstration to show the differences between elements. Propan-2-ol gives yellow ppt of iodoform whereas methanol does not.
Ethanol when reacted with I2 and NaOH or NaOI gives yellow ppt of iodoform since it has the presence of CH3-CH OH- group. Here we will discuss the concept in. JEE Mains Chapter wise Practice Questions Last 30 Years with 5000 Questions for online practice.
O-nitrophenol is more volatile. LDPE Chemical Compatibility Chart. Check the chemical compatibility of LDPE low density polyethylene with various chemicals solvents alcohols and other products.
Haloalkanes And Haloarenes Class 12 NCERT Solutions includes all the important topics with detailed explanation that aims to help students to understand the concepts better. Students who are preparing for their Class 12 exams must go through NCERT Solutions for Class 12 Chemistry Chapter 10 Haloalkanes and HaloarenesGoing through the solutions provided on this page will. B From alkyl halide and sodium alkoxide.
Short Answer Type Questions I 2 Marks Question 23. Chromatography separates substances on the basis of differential adsorption of compounds to the adsorbent and compounds move through the column at different rates enabling them to separate into fractions. Which of the following isomers is more volatile.
It is not reactive at room temperatures except by strong oxidizing agents and some solvents cause swelling. Highly resistant to most acids alcohols detergents and solvents. Maintains high strength toughness and self-lubrication as low as 5K -268C -450F and good flexibility at 194K -79C -110F.
2014 Very Short Answer Type Questions 1 Mark Question 21. Add 1ml of 1 iodine solution to it. Nomenclature methods of preparation physical and chemical properties of primary alcohols only identification of primary secondary and tertiary alcohols mechanism of dehydration uses with special reference to methanol and ethanol.
The reaction of iodine a base and a methyl ketone gives a yellow precipitate along with an antiseptic smellIt also tests positive for a few specific secondary alcohols that contain at least one methyl group. Iodoform test is used to check the presence of carbonyl compounds with the structure R-CO-CH 3 or alcohols with the structure R-CHOH-CH 3 in a given unknown substance. Iodoform also known as triiodomethane and inaccurately as carbon triiodide is the organoiodine compound with the chemical formula C H I 3.
To illustrate the differences in reactivity of isomeric alcohols try using the iodoform demonstration in the video. The melting point of PTFE 1 is 600K 327C 620F Low temperature. Ideal for use with reactive and corrosive chemicals.
Before permanent installation test the equipment with the chemicals and under the specific conditions of your application. This test is given by secondary alcohols ketones and acetaldehyde. LDPE is defined by a density range of 09100940 gcm3.
In chemistry column chromatography implies a method that is used to isolate a chemical compound from a mixture. Organic Compounds with Functional Groups Containing Oxygen and Nitrogen. Take 1ml of given organic compound in a clean dry test tube.
Here the alkyl halide should be primary and alkoxide should be tertiary. The reaction between zinc powder and sulfur. Effect of substituents on alpha- carbon on.
A pale yellow crystalline volatile substance it has a penetrating and distinctive odor in older chemistry texts the smell is sometimes referred to as that of hospitals where the compound is still commonly used and. Electronic structure important methods of preparation important reactions and physical properties of alcohols phenols ethers aldehydes ketones carboxylic acids nitro compounds amines diazonium salts cyanides and isocyanides Specific.

A Test To Distinguish Between Ethanol And Methanol Experiment Rsc Education

Iodoform Test Description And Mechanism Compounds That Test Positive

Iodoform Test Description And Mechanism Compounds That Test Positive
No comments for "Iodoform Test for Alcohols"
Post a Comment